A Molecular Compound Could Contain Which of the Following Atoms
The empirical formula expresses the smallest whole-number ratio of the atoms in the molecule. The molecular formula of octane is C 8 H 18.

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube
5th - 6th grade.

. The whole number of atoms are equal for each element. These molecular compounds covalent compounds result when atoms share rather than transfer gain or lose electrons. Molecules of alcohol A contain a branched carbon chain and can be oxidized to an aldehyde.
When writing the chemical formula the element that is the least electronegative the element that is farther left or further down within the same family group is written first while the more electronegative element is. 1 Number of carbon atoms 2 The type of bond between the carbon atoms. None of these it is not possible for a formula to be both empirical.
The molecular structure of the methane molecule CH 4 is shown with a tetrahedral arrangement of the hydrogen atomsVSEPR structures like this one are often drawn using the wedge and dash notation in which solid lines represent bonds in the plane of the page solid wedges represent bonds coming up out of the plane and dashed lines represent bonds going down into the plane. Hydrocarbons that contain the maximum number of hydrogen atoms per carbon atom. The molecular formula indicates the exact number of atoms in the molecule.
Covalent bonding is an important and extensive concept in chemistry and it will be treated in considerable detail in a later chapter of. Check all that apply. Two or more elements bonded together through ionic attraction.
The remainder of the mass was due to oxygen. A Molecule containing only carbon hydrogen bonded together by covalent bonds. And molecules of alcohol C can be oxidized to neither an aldehyde nor a ketone.
Determine whether the following atoms will form an ionic compound or a molecular compound and give the formula of the compound. Total of 12 valence electrons in the molecule. Many compounds do not contain ions but instead consist solely of discrete neutral molecules.
Contain at least one carbon-carbon double bond. All compounds are molecules but not all molecules are compounds. Four ceH atoms 4 times 1 4 valence electrons.
The compound could contain a CO double bond The compound could contain a CC triple bond The compound could contain a cyclopropane ring The compound could contain a. A molecule of a covalent compound contains three atoms of fluorine F and one atom of boron B. A molecular compound could contain which of the following atoms.
Compounds contain two or more atoms and are held together by ionic bonds whereas molecules are held together by covalent bonds. If the relative molecular mass of the compound is 60 calculate i the empirical formular of x ii the molecular formular of x. C 6 H 6.
C 2 H 6 O. In the above case the empirical formula of glucose is rmCrmH_2rmO. A molecular compound known to contain only the elements carbon hydrogen and oxygen was analyzed and found to contain 3870 carbon and 974 hydrogen by mass.
The formula HCN shows that hydrogen cyanide could contain 2 atoms of each element. Every compound is a molecule but not every molecule is a compound. A molecule of octane which is a component of gasoline contains 8 atoms of carbon and 18 atoms of hydrogen.
Contain at least one carbon-carbon triple bond. A molecule is a substance that contains two or more atoms chemically joined such as H 2O2. The carbon atoms are bonded together with each carbon also bonded to two hydrogen atoms.
OK z I Do you know the answe. Which box contains a compound of carbon and hydrogen. Alcohols A B and C all have the composition C 4 H 10 O.
The molecular formula for glucose is rmC_6rmH_12rmO_6. OK z I Do you know the answe. Two ceC atoms 2 times 4 8 valence electrons.
It was determined that the compound had a. Molecules of alcohol B contain a linear carbon chain and can be oxidized to a ketone. Write the Lewis structures of these molecules.
Hydrocarbons differ from each other in two ways. CH4 HCH3 H4C H2CH2 1 See answer Advertisement Advertisement victorialara1k is waiting for your help. An organic compound x contains 40 carbon 67 hydrogen the third compound oxygen.
One element shows only one atom so the formula ratio could not be further reduced but the molecular formula could just have one atom. Check all that apply. One molecule of the compound contains five carbon atoms One molecule of the compound contains two oxygen atoms.
Add your answer and earn points. The bonds holding the molecule together are caused by the sharing of electrons. Organic compounds that contain double or triple carbon-carbon bonds.
What is the correct molecular formula for a compound that contains one atom of carbon and four atoms of hydrogen. The formula HCN indicates that a molecule of hydrogen cyanide contains one hydrogen atom one carbon atom and one nitrogen atom. What is the correct molecular formula and chemical name of the compound described in this statement.
Possible and still retain the identity of the molecule. I know it Think so Unse No idea. A molecule is made of one carbon atom and two oxygen atoms.
Hydrocarbons are an example of some of the more simple organic compounds since they only contain carbon hydrogen. Atoms elements molecules compounds and mixtures. A compound is a substance that is made up of two or more different elements that are chemically joined such as H 2OCON aCl.
A sodium and chloride b carbon and 4 hydrogen c magnesium and bromine. There are many types of organic molecules. The atoms within a compound also must be different from eachother whereas a molecule can consist of only one element.
A molecular compound could contain which of the following atoms. Consider the compound ethene which has a molecular formula of ceC_2H_4.
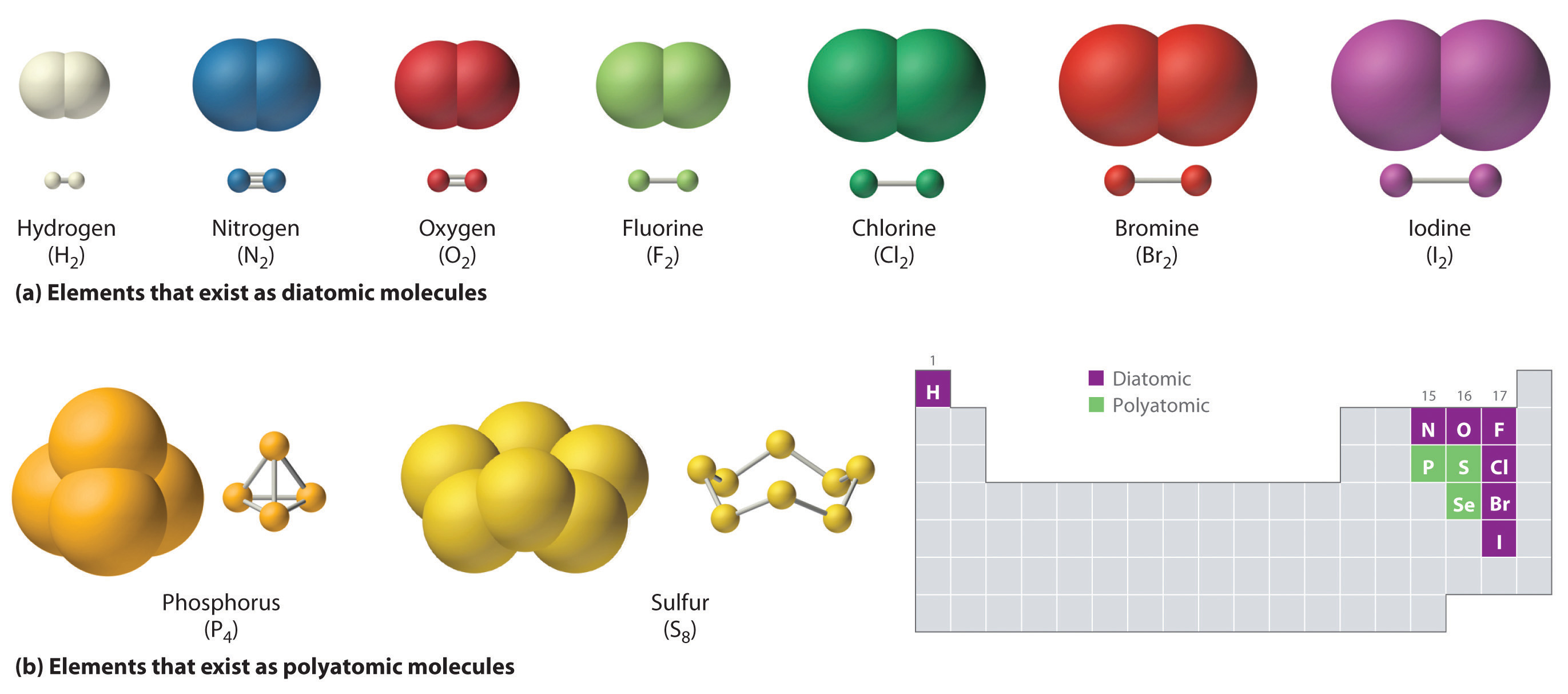
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube
0 Response to "A Molecular Compound Could Contain Which of the Following Atoms"
Post a Comment